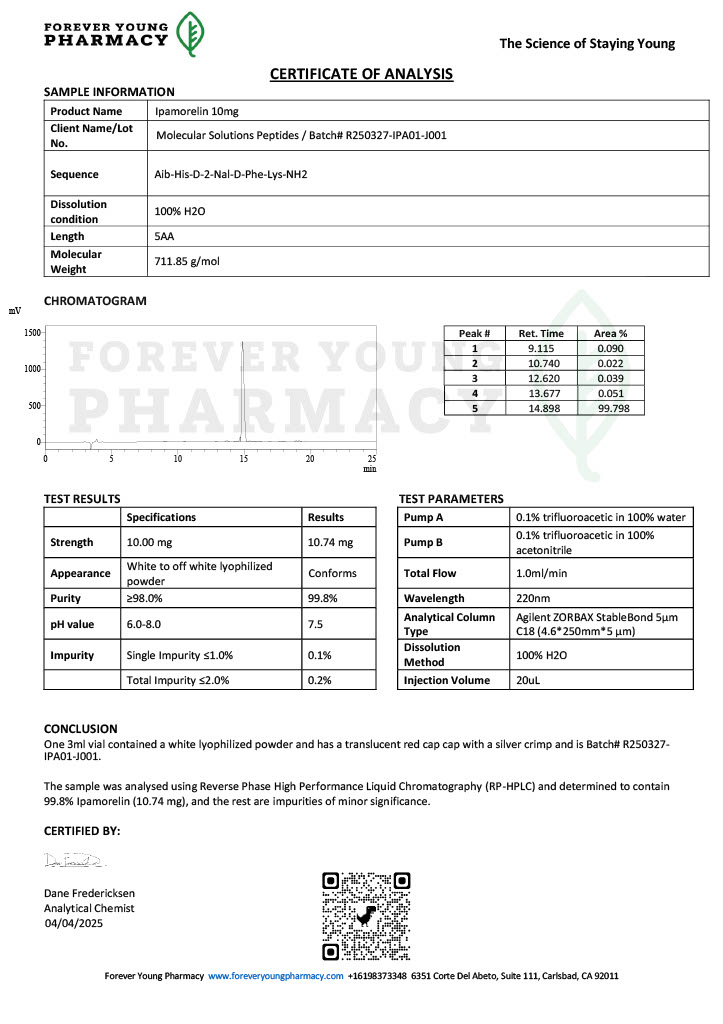
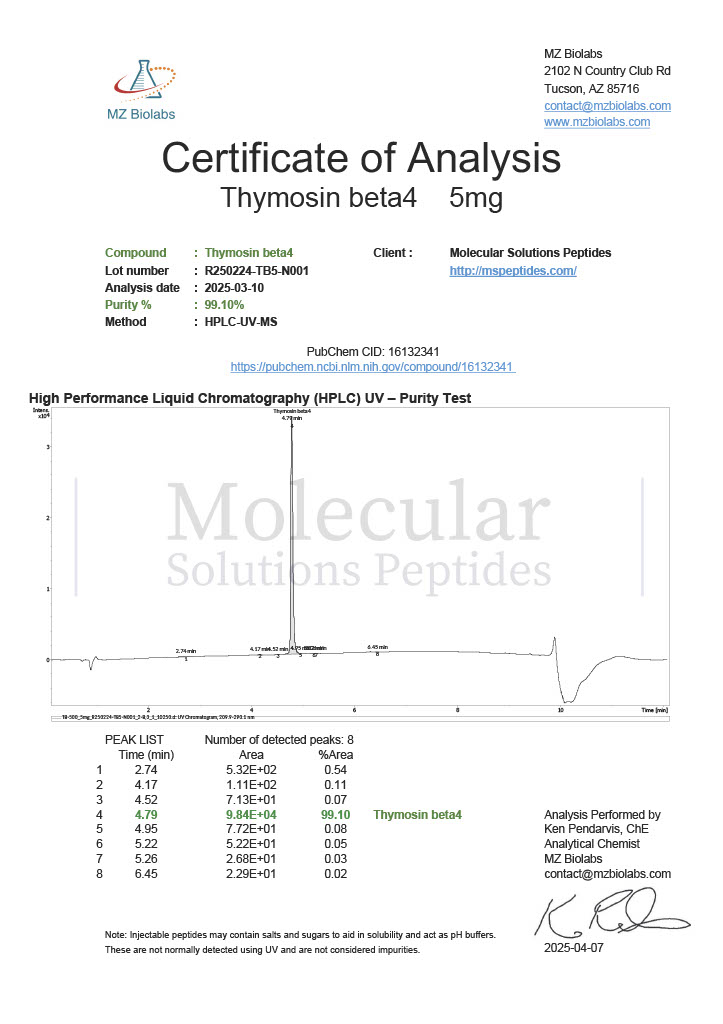
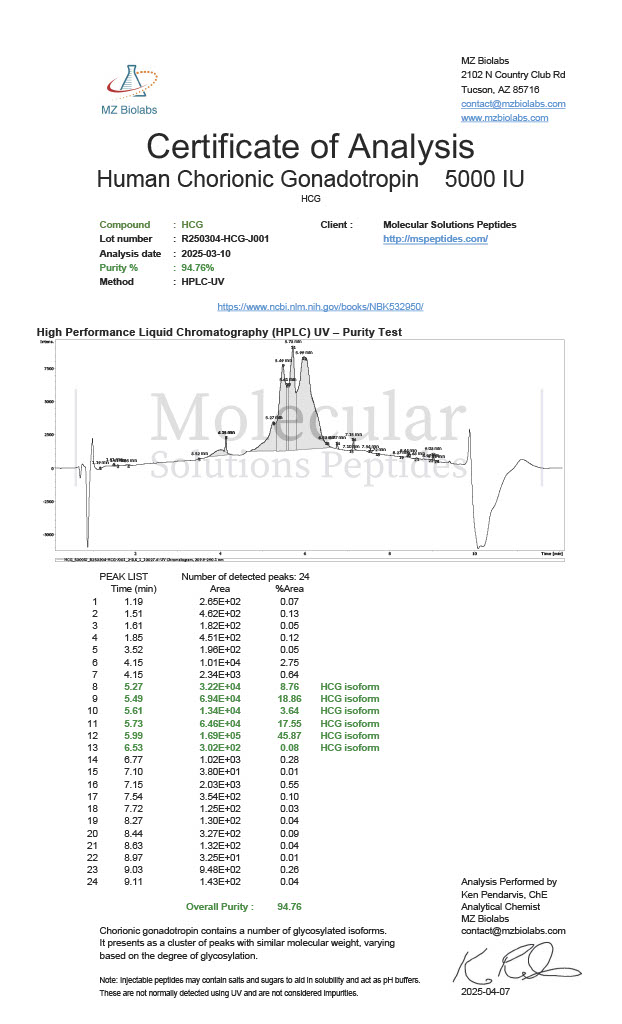
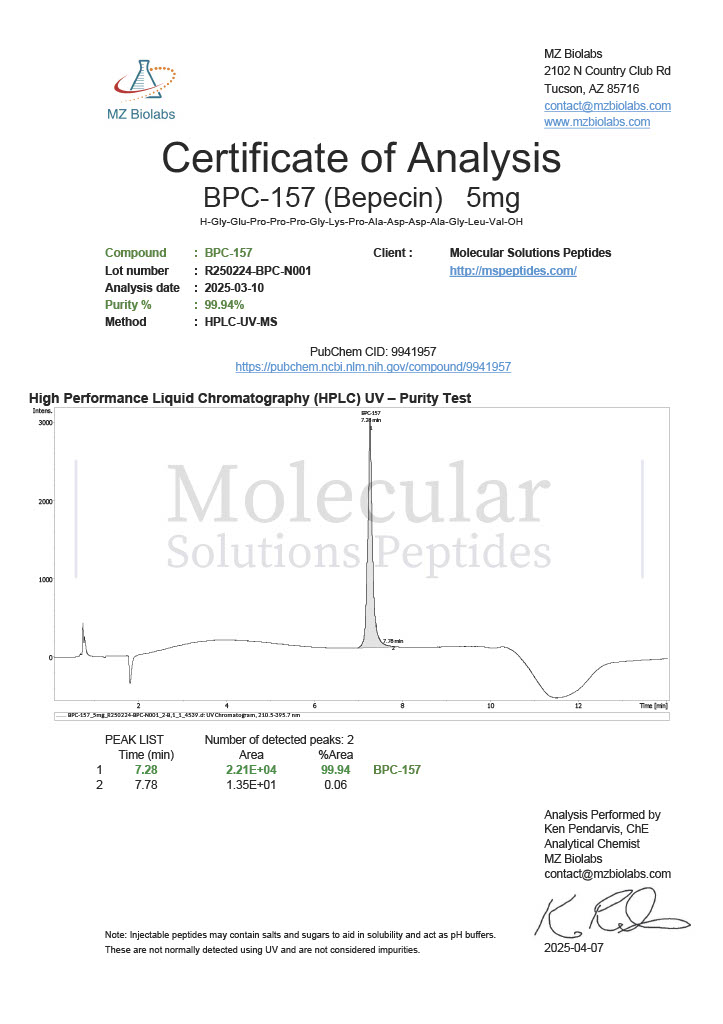
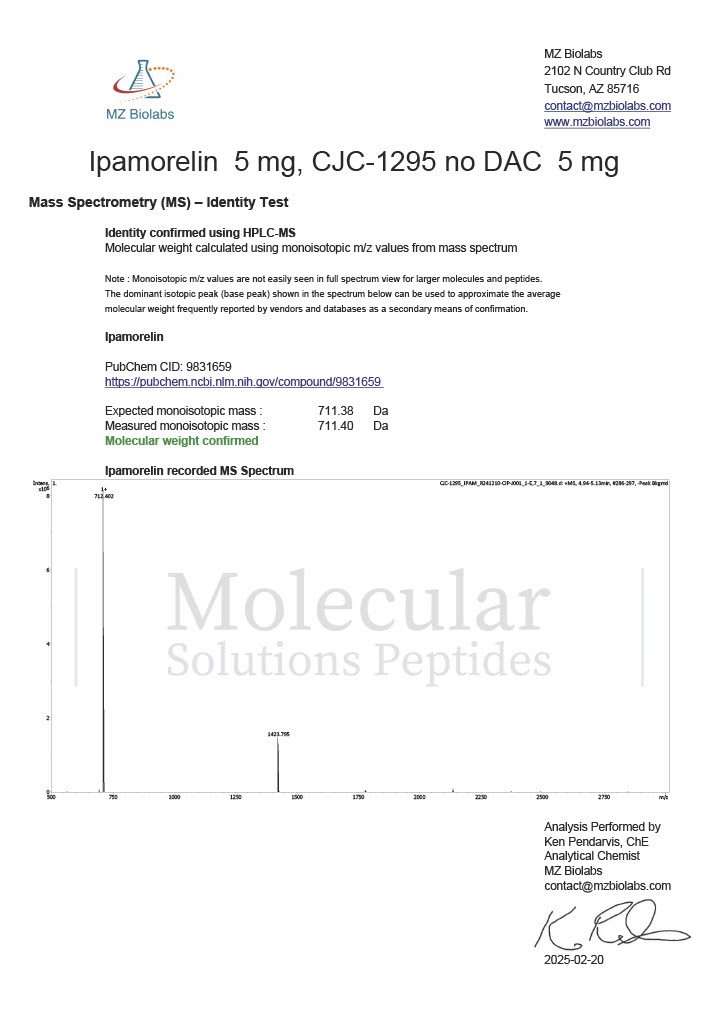
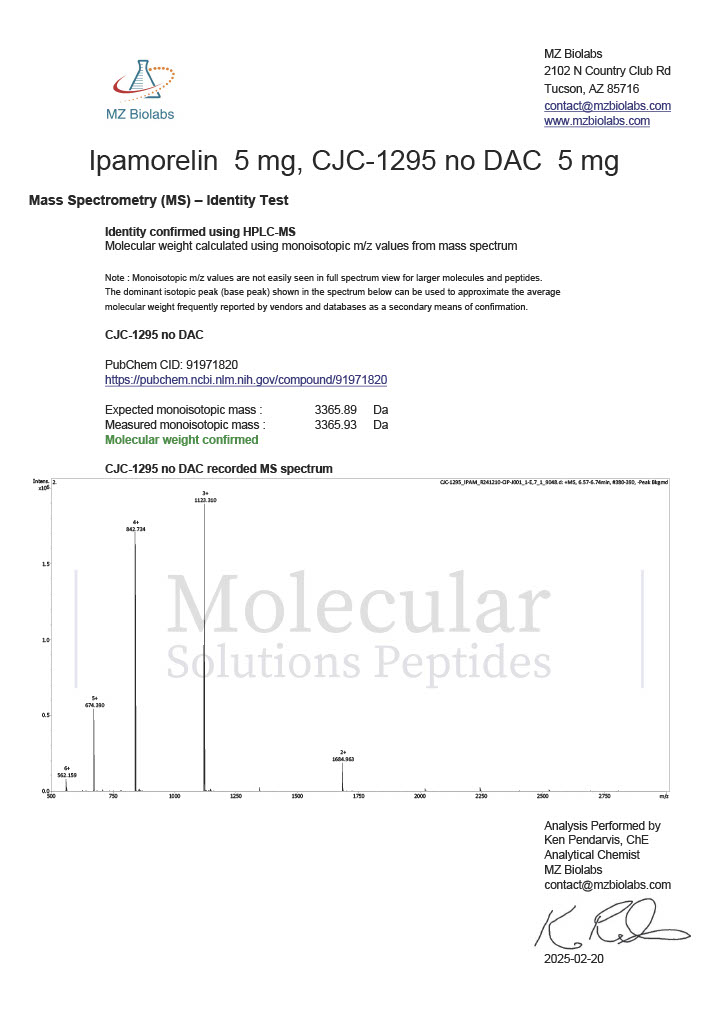
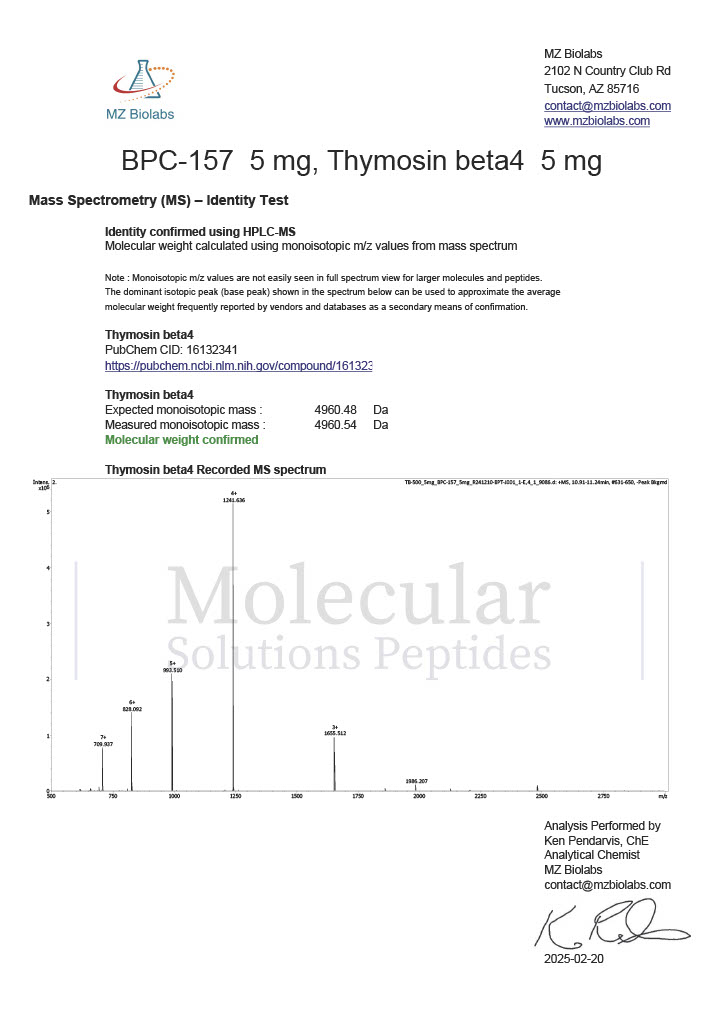
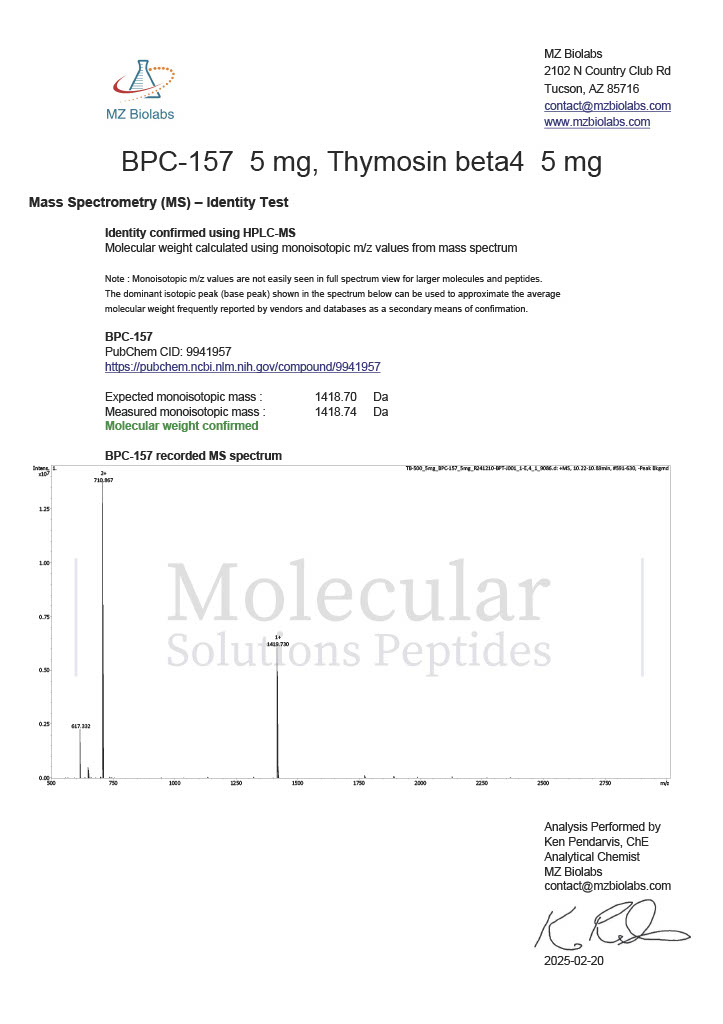
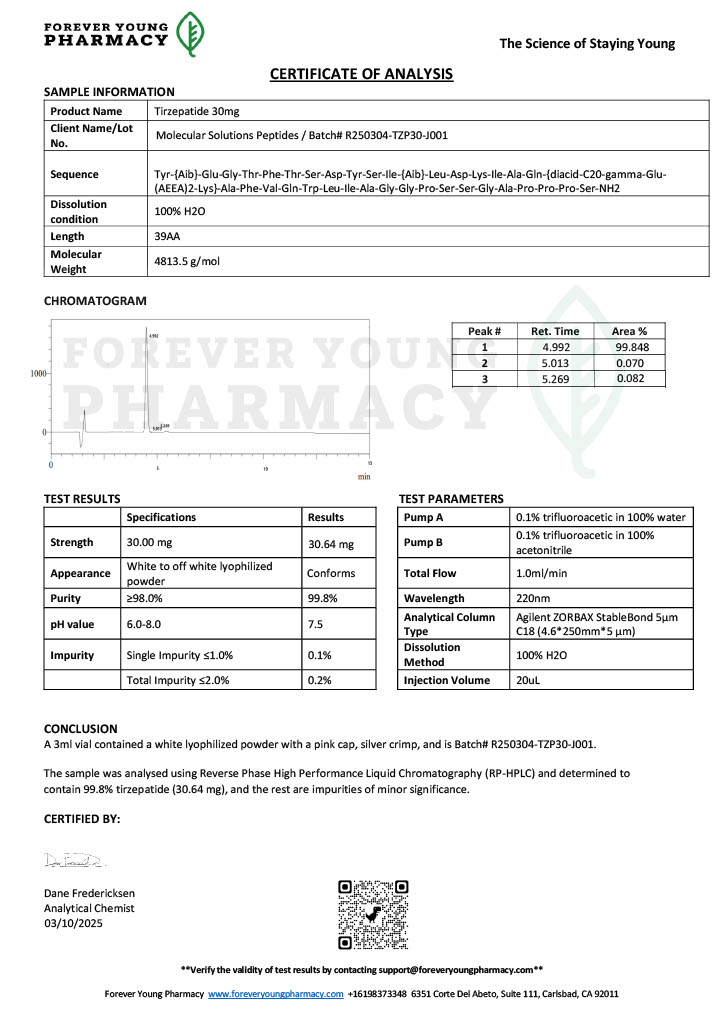
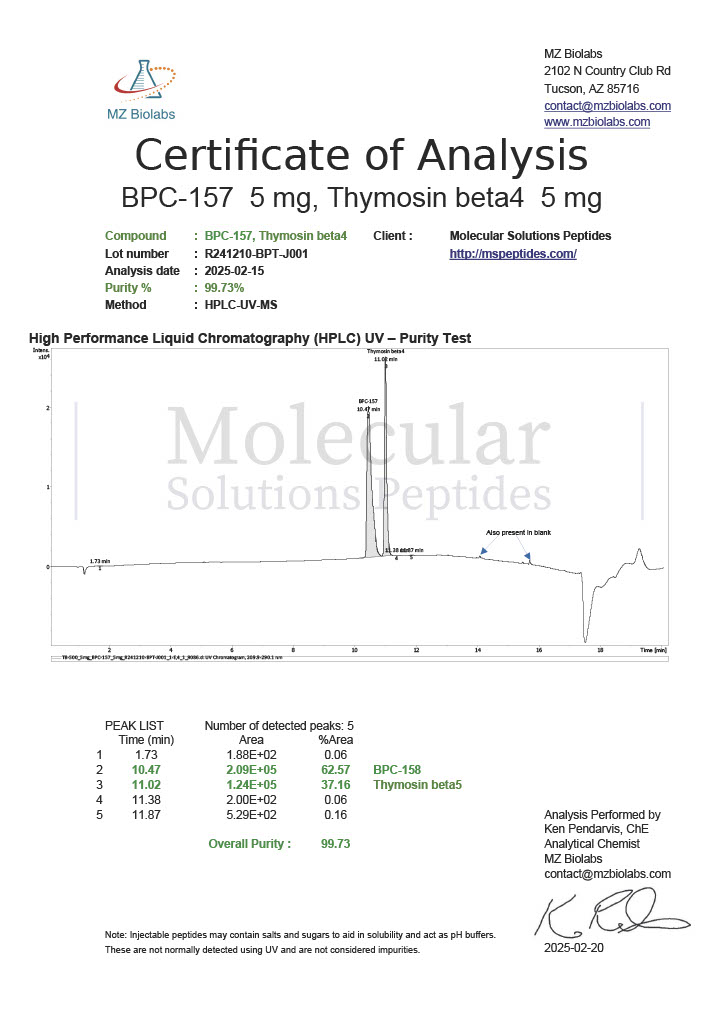
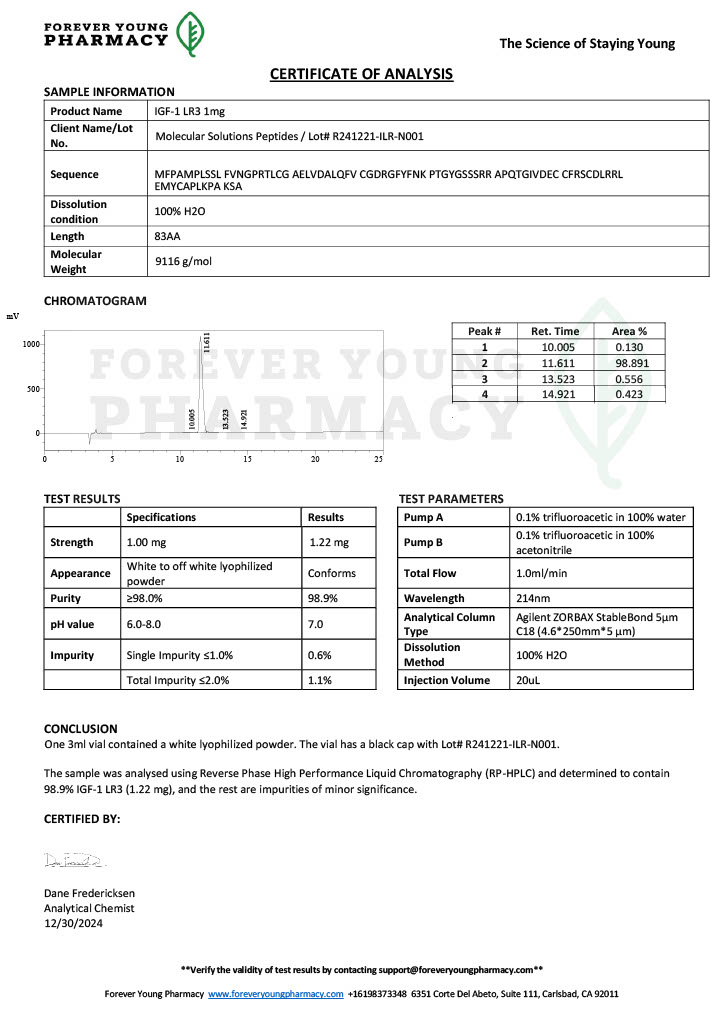
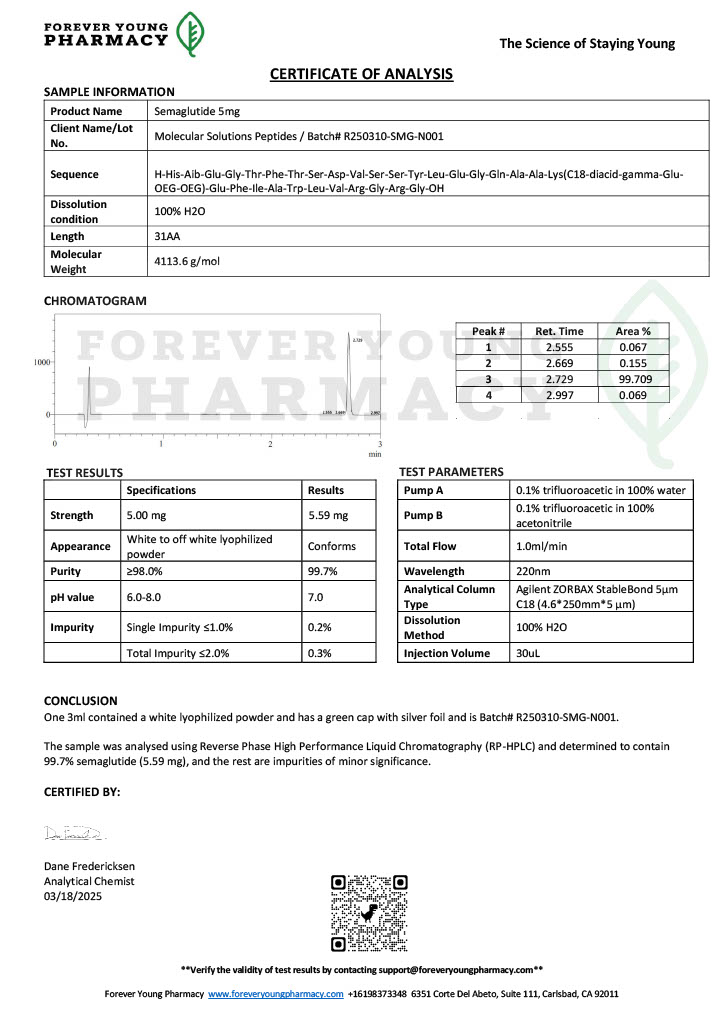
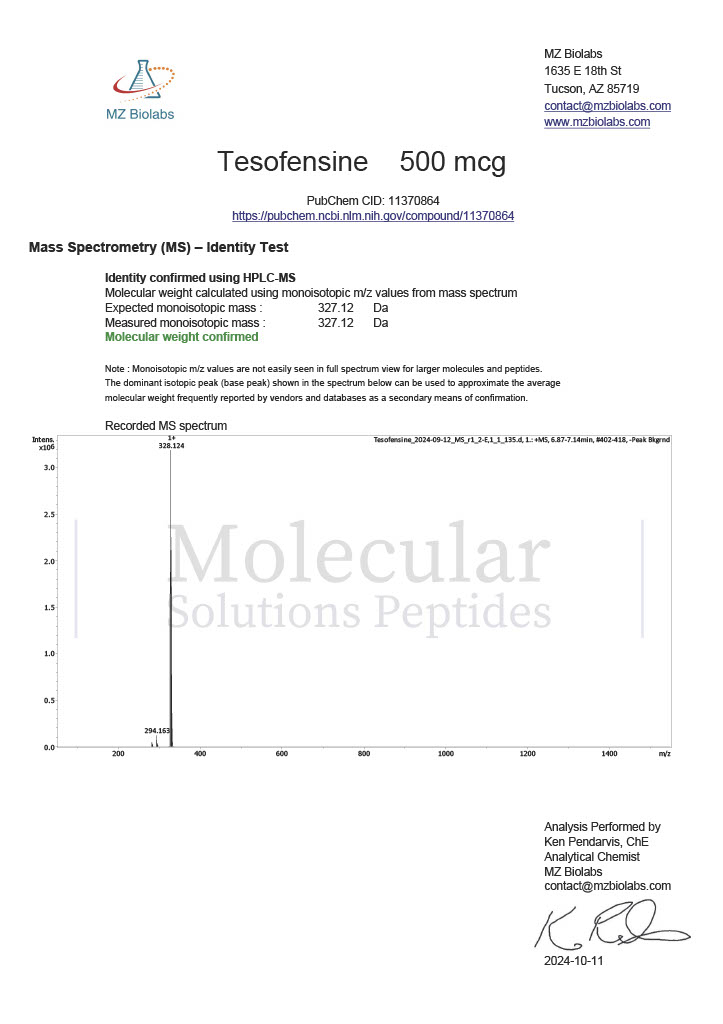
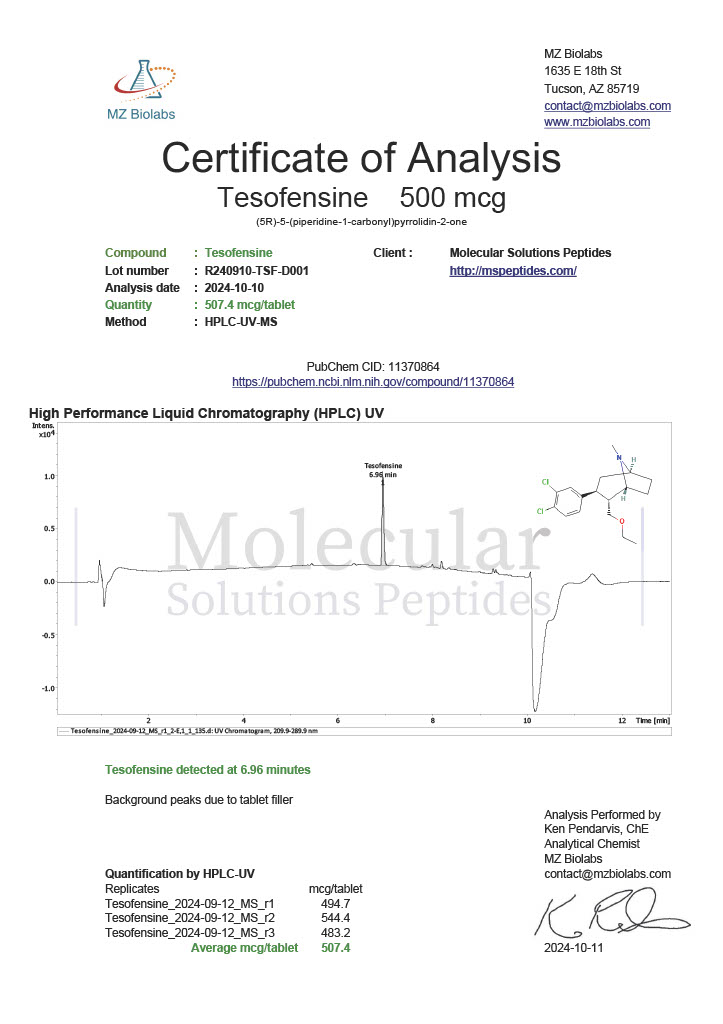
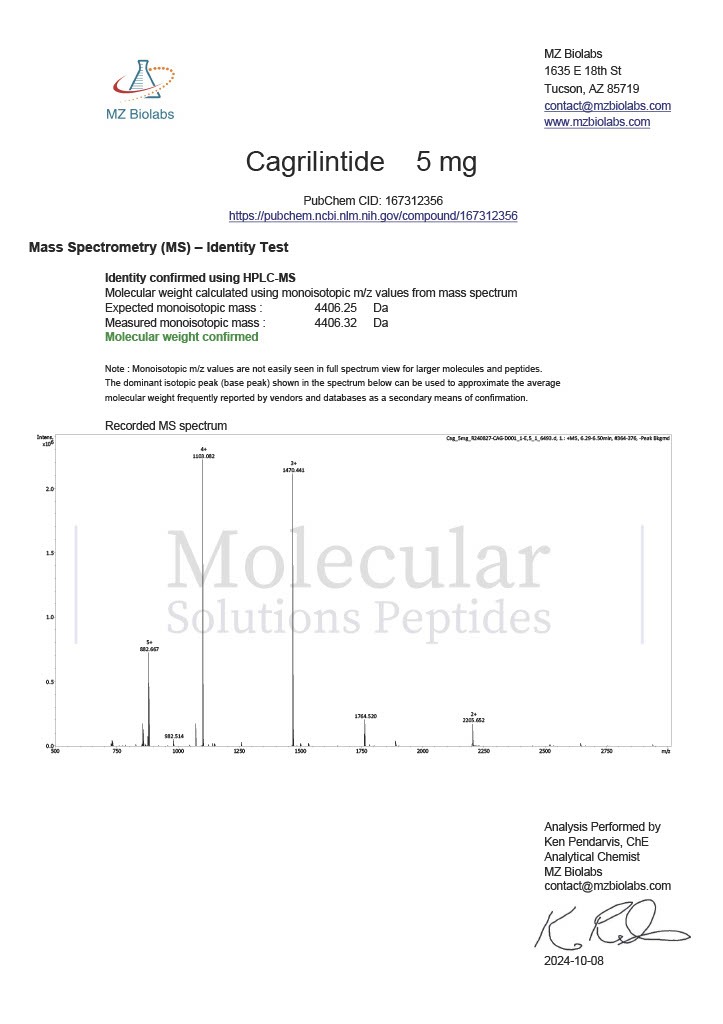
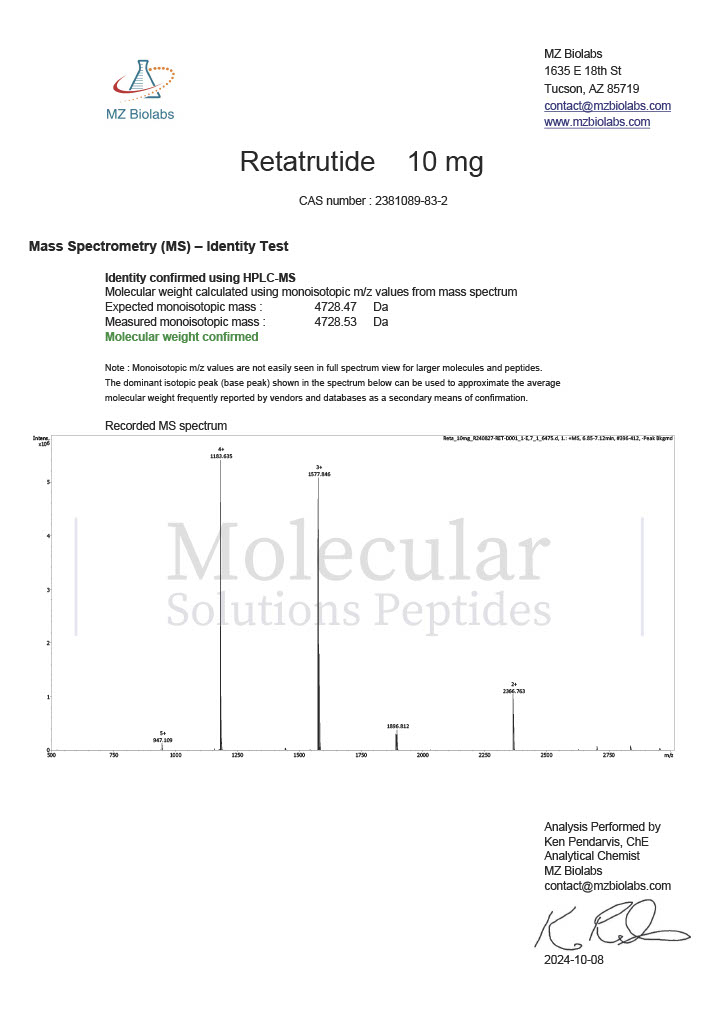
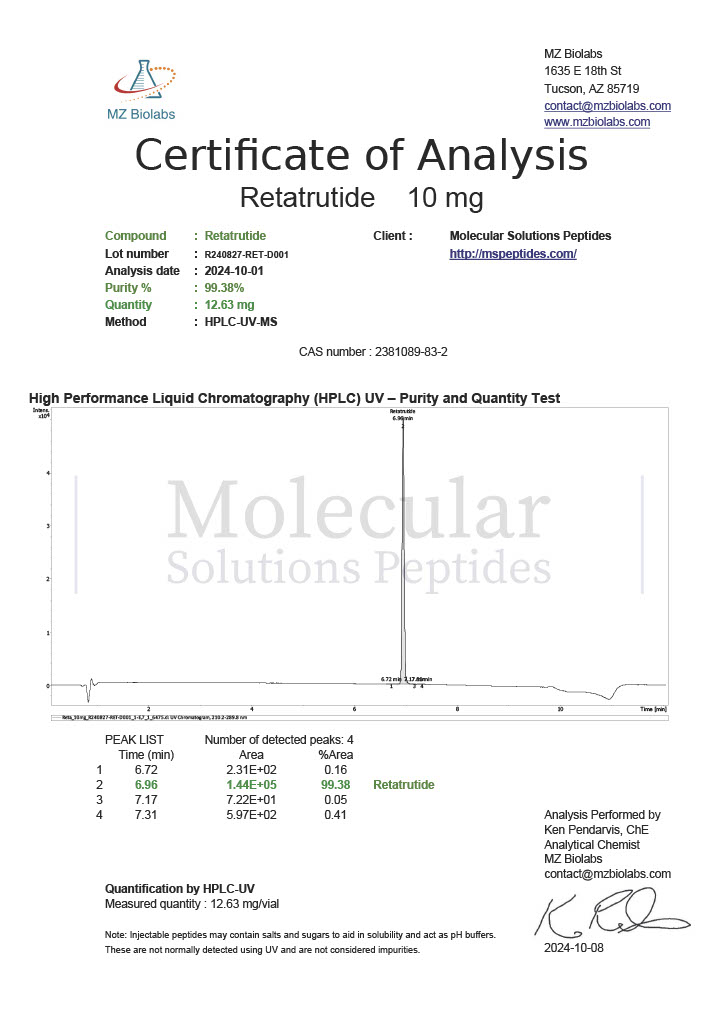
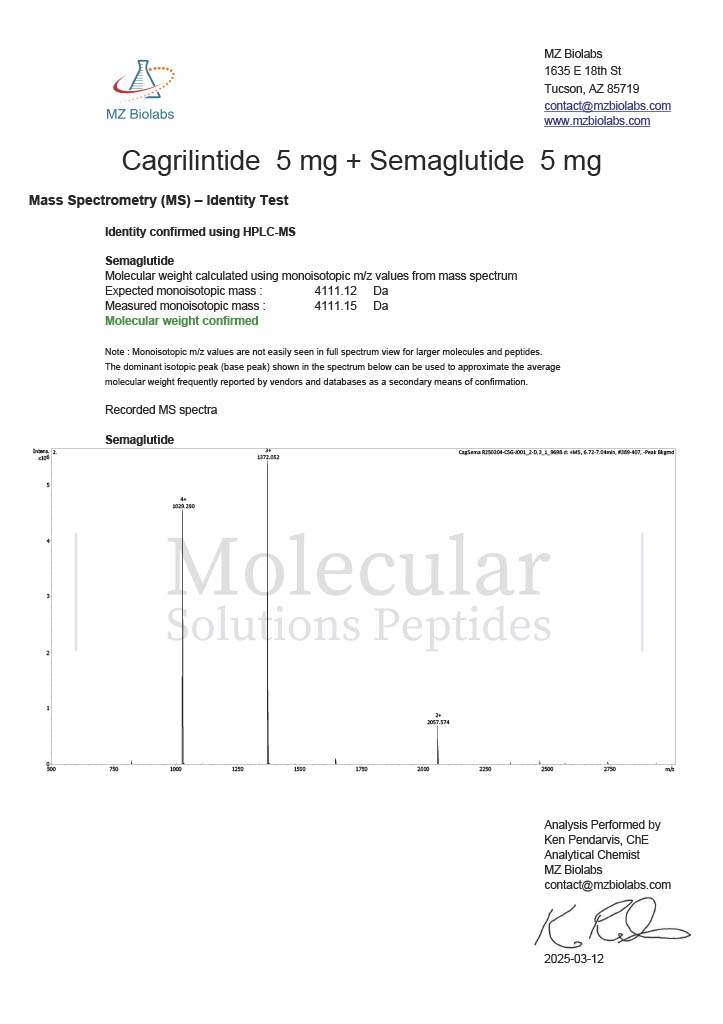
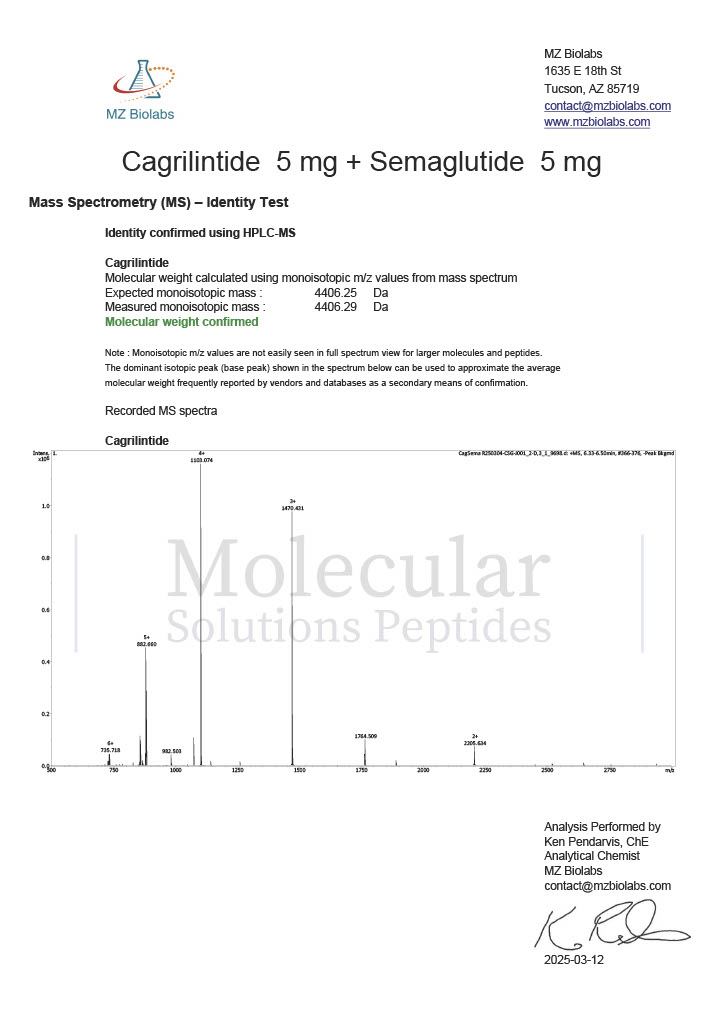
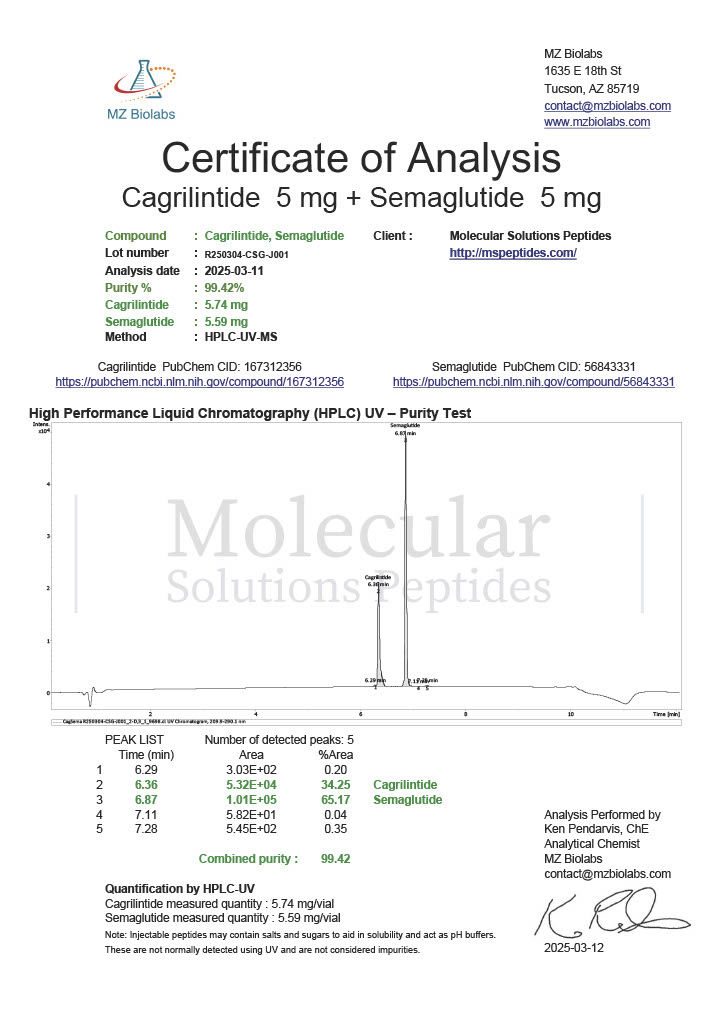
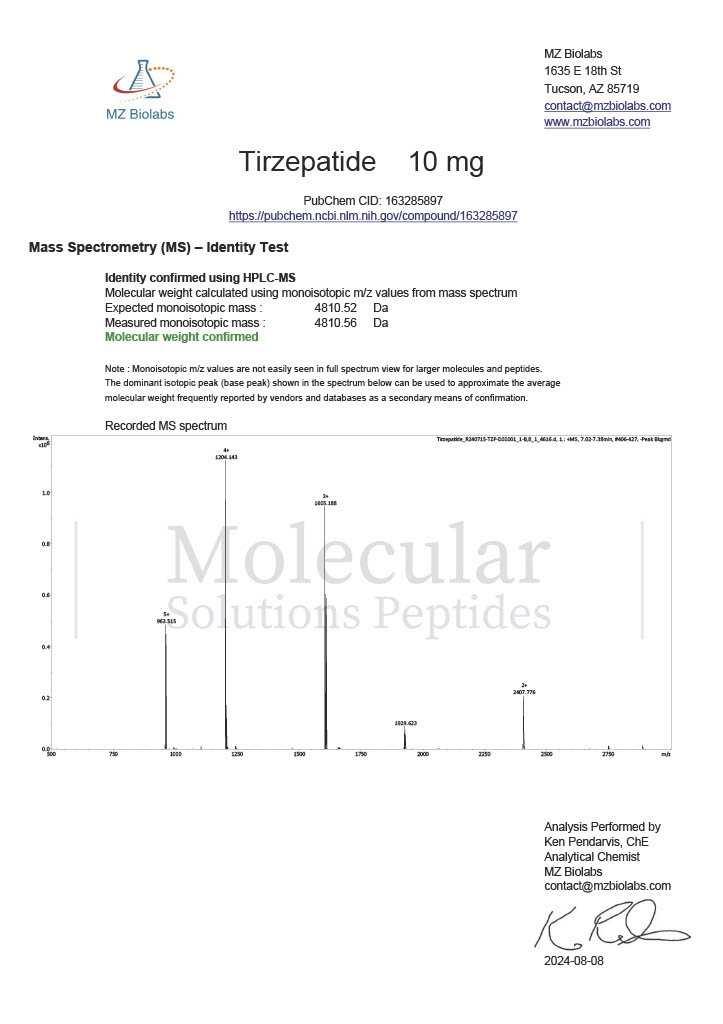
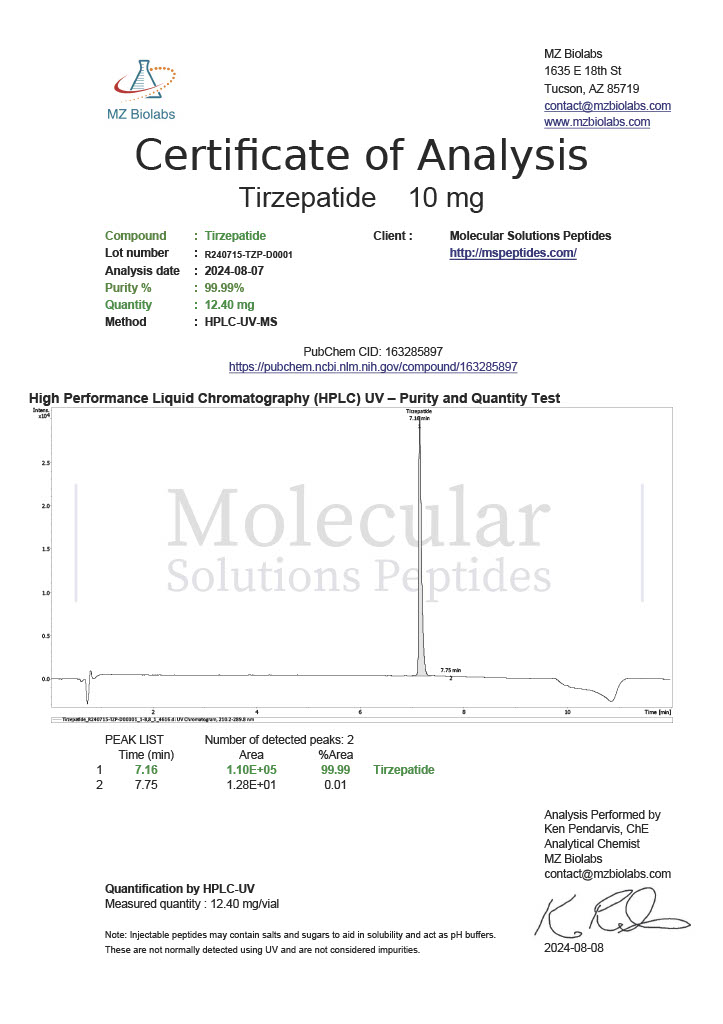
Peptide research continues to play a central role in biochemistry, molecular biology, and pharmaceutical development. At the foundation of this work are peptides themselves, short chains of amino acids linked by peptide bonds. While the term peptide is broad, researchers often distinguish between base peptides and modified peptides.
Understanding the differences between these two categories is critical for experimental design, reproducibility, and interpretation of results. This discussion focuses strictly on peptides from a research perspective and does not advocate or imply human use.
Understanding Base Peptides in Research
Base peptides, sometimes referred to as unmodified or native peptides, are peptide sequences composed solely of standard amino acids arranged in a specific order. They represent the fundamental structure of a peptide without chemical alterations, conjugations, or functional enhancements.
In research settings, base peptides are often used as starting materials. They provide a clean and well defined sequence that can be studied for intrinsic properties such as folding behavior, binding affinity, enzymatic susceptibility, and structural stability. Because they lack additional functional groups or chemical tags, base peptides allow researchers to observe how the primary amino acid sequence behaves on its own.
Base peptides are also frequently employed as control materials. When evaluating how a specific modification affects activity or stability, comparing results to the corresponding base peptide is essential. This comparison helps isolate the effect of the modification rather than confounding variables.
What Are Modified Peptides?
Modified peptides are derived from base peptides through intentional chemical or structural changes. These modifications can occur at various points in the peptide chain and may involve adding functional groups, altering amino acids, or attaching external molecules.
Common types of peptide modifications include terminal capping, amino acid substitutions, cyclization, PEGylation, and the attachment of labels such as fluorescent markers or affinity tags. Each modification serves a specific experimental purpose. Some aim to increase stability, others improve detectability, and some enhance interaction with a target molecule in a controlled research environment.
Unlike base peptides, modified peptides are not meant to represent the simplest form of a sequence. Instead, they are engineered tools designed to answer specific research questions.
Structural Differences Between Base and Modified Peptides
The most obvious difference between base peptides and modified peptides lies in their chemical structure. Base peptides consist only of amino acids connected by peptide bonds, with free or naturally terminated ends. Their structure reflects the direct translation of a defined amino acid sequence.
Modified peptides introduce additional elements that alter this structure. For example, adding an acetyl group to the N terminus changes the charge and polarity of the peptide. Cyclization links the ends or side chains together, restricting conformational flexibility. Labeling introduces bulky groups that can influence folding or interaction with solvents.
These structural differences can significantly impact how peptides behave during experiments. Even small modifications can change solubility, aggregation tendencies, or binding kinetics. For this reason, understanding the base structure first is often necessary before meaningful interpretation of modified peptide data.
Functional Implications in Experimental Design
Base peptides and modified peptides serve different but complementary roles in experimental workflows. Base peptides are typically used to establish baseline behavior. They allow researchers to observe natural interactions, degradation pathways, and conformational preferences under controlled conditions.
Modified peptides are introduced when a specific experimental challenge needs to be addressed. For instance, if a base peptide degrades too quickly in an assay system, a stabilized version may be synthesized for extended observation. If detection is difficult, a labeled peptide may be used to track localization or binding events.
The key point is that modified peptides are tools built upon the foundation of base peptides. Without understanding the behavior of the base sequence, it becomes difficult to interpret the results obtained from modified versions.
Reproducibility and Data Interpretation
Reproducibility is a cornerstone of scientific research. Base peptides are often favored when reproducibility is a priority because their simplicity reduces variability. Fewer chemical components mean fewer potential sources of unexpected interactions or side reactions.
Modified peptides, while powerful, introduce additional variables. A modification may alter how a peptide interacts with solvents, surfaces, or other molecules in the system. These changes can sometimes lead to results that are not directly comparable across different studies or laboratories.
For this reason, many researchers report data for both base peptides and modified peptides. This dual reporting provides context and allows others to better understand how modifications influence outcomes. It also supports more robust conclusions by linking observed effects back to the original peptide sequence..
Why the Distinction Matters in Research Outcomes
The distinction between base peptides and modified peptides is not merely academic. It directly impacts how experimental results are interpreted and communicated. When a study reports findings based on a modified peptide without reference to the base sequence, it can be difficult for others to assess the broader relevance of the data.
Base peptides provide a common reference point across studies. They allow researchers to build upon each other’s work by starting from the same fundamental sequence. Modified peptides then extend this knowledge by exploring how specific changes influence behavior in controlled ways.
Clear differentiation between these two types of peptides also supports transparency. Readers can better understand what was tested, why it was tested, and how the results relate to the underlying biology or chemistry being investigated.
Choosing Between Base and Modified Peptides
Selecting whether to use a base peptide or a modified peptide depends on the research question. Early stage studies often benefit from starting with base peptides to establish foundational knowledge. As hypotheses become more refined, modified peptides can be introduced to probe specific mechanisms or overcome technical limitations.
Importantly, this choice should always be documented clearly in research records and publications. Precise descriptions of peptide composition help ensure that experiments can be replicated and that conclusions are drawn appropriately.
Base peptides and modified peptides each play a vital role in peptide research, but they serve different purposes. Base peptides offer simplicity, clarity, and a reliable foundation for understanding intrinsic sequence behavior. Modified peptides provide versatility and functionality, enabling researchers to explore complex questions that would be difficult to address otherwise.
Recognizing the differences between these two categories is essential for sound experimental design, accurate data interpretation, and meaningful scientific communication. By treating base peptides as the reference point and modified peptides as targeted tools, researchers can generate more robust and reproducible insights into peptide structure and function, all within a strictly research focused framework.
Disclaimer: All products on this site are for Research, Development use only. Products are Not for Human use of any kind. The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease.